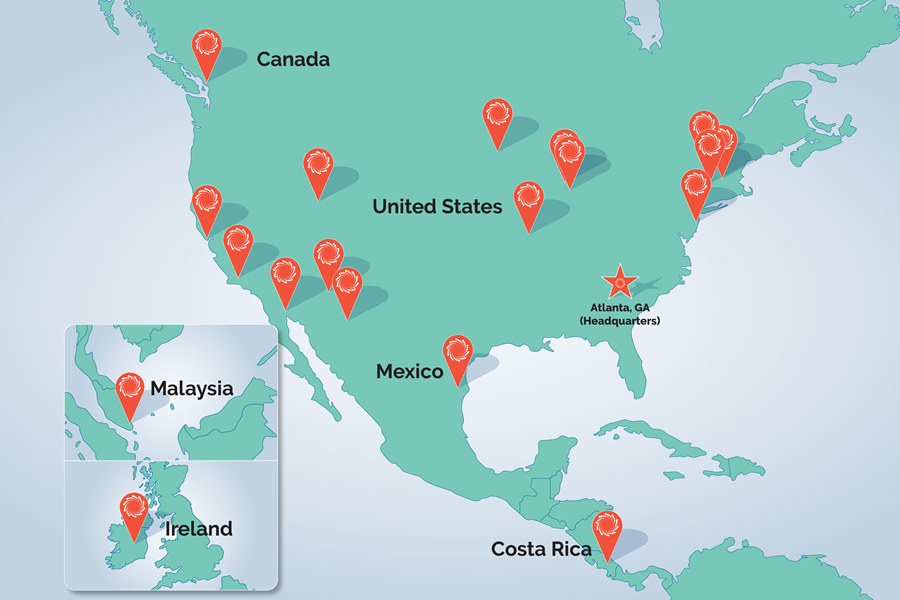
Cleanroom Injection Molding
Cleanrooms are critical to the injection molding process. Learn what a cleanroom is, how it’s used, why the standards of a cleanroom are essential, and how Spectrum Plastics Group Minneapolis’s cleanroom supports the success of our projects.