Advanced Additive Manufacturing
Spectrum has developed groundbreaking additive manufacturing technologies, utilizing proprietary processes and medical-grade materials to create components with complex tolerances, geometries, and features.

Advanced Additive Manufacturing
Spectrum has developed groundbreaking additive manufacturing technologies, utilizing proprietary processes and medical-grade materials to create components with complex tolerances, geometries, and features.

Additive manufacturing (AM), also known as 3D printing, is an innovative suite of advanced technologies that enables the fabrication of medical components and products with remarkably complex and high-resolution features which often cannot be achieved within the constraints of conventional manufacturing methods. Compared to traditional subtractive machining methods, AM is more efficient and can deliver functional prototypes into the hands of medical engineering teams within a matter of days, instead of weeks. Spectrum has developed groundbreaking, proprietary AM technologies for creating medical products that the competition cannot, such as high-precision, medical-grade single and multi-lumen tubing—a first in the industry.
Additive manufacturing is a rapidly advancing field as new technologies, equipment, and specialized materials continue to enter the market. Spectrum’s industry-leading AM capabilities enable more creative design, innovative rapid prototyping, and seamless transition to high-volume manufacturing.
Benefits of Spectrum’s additive manufacturing capabilities and services include:
Spectrum’s advanced additive manufacturing (AM) technologies produce high-precision components for medical devices, which continue to get smaller and more complex. Our primary AM methods are:

This technology is ideal for creating ultra-precise micro medical device components, such as hubs and luers, layer-by-layer. These high-detail, tight-tolerance micro parts can have features as small as .0002 inches, fabricated in crisp detail. Parts can be made from a wide variety of medical-grade biocompatible or non-medical-grade photopolymer resins.

Stereolithography (SLA) is a popular 3D-printing method that produces a variety of high-precision prototypes and end-use parts from a range of advanced materials with fine features and smooth surface finishes. Common products are hubs, luers, handles, and larger components, all with complex features and geometries. SLA is used to make customized profile devices with individualized fits from medical-grade biocompatible or non-medical-grade photopolymer resins.

Spectrum’s engineering teams have developed groundbreaking AM technologies that utilize proprietary processes and medical-grade materials to create components with complex tolerances, geometries, and features. One of these methods can produce high-precision, medical-grade tubing (single and multi-lumen tubing) that is not extrusion-based—a first in the industry. Other products include catheter tips, hubs, dilators and sheaths, and customized profile devices—all from various medical-grade materials.

FDM or fused deposition modeling is the process of creating objects by building up layers through the extruding material process. Spectrum uses custom additive manufacturing technologies to process all medical-grade thermoplastic materials with high-resolution and tight-tolerance parts. These include Nylon, Pebax®, polypropylene, polycarbonate, acrylonitrile butadiene styrene (ABS), polylactic acid (PLA), PEEK, polyetherketone (PEK), high impact polystyrene (HIPS), urethanes, and custom materials.

SLS, or Selective Laser Sintering, is a 3D printing process that uses a high-powered laser to fuse thermoplastic powders into a solid object. Some commonly used materials are Nylon 12, Nylon 11, and TPUs. This process can quickly produce high-quantity and high-quality parts.
Silicone 3D printing is a technology that allows the fabrication of parts that are highly flexible and durable out of silicone materials. This process allows for a wide range of medical-grade and non-medical-grade silicones. What is also unique about this process is the ability to print very low durometer silicones, such as Shore 10A.
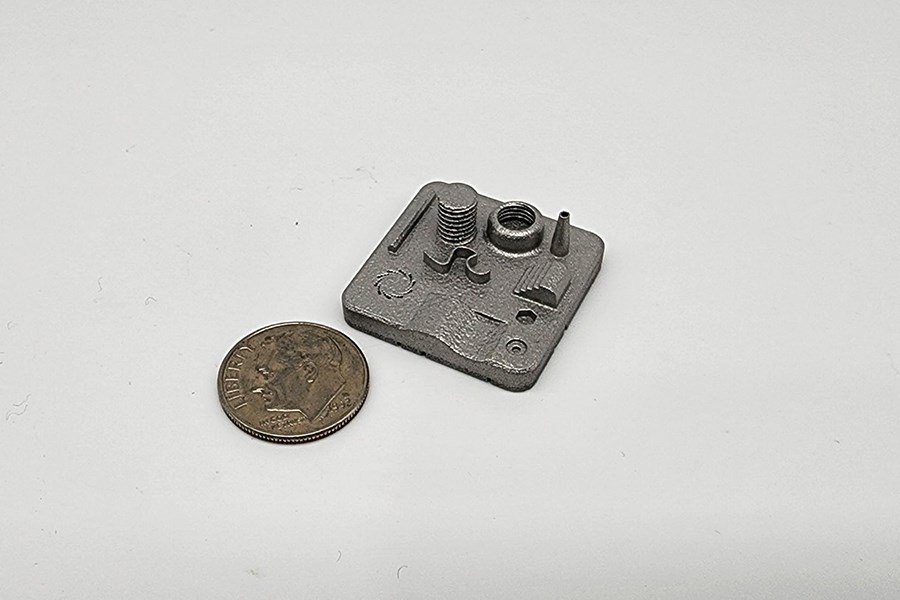
Metal SLM (Selective Laser Melting) is a 3D printing process that uses a high-powered laser to melt or weld metallic powder layer by layer into a solid object. Spectrum utilizes stainless steel powders to create high-precision, isotropic metal components and fixtures that cannot be made with conventional methods.
Spectrum uses additive manufacturing technologies to process all medical-grade thermoplastic materials. These include Nylon, Pebax®, polypropylene, polycarbonate, acrylonitrile butadiene styrene (ABS), polylactic acid (PLA), PEEK, polyetherketone (PEK), high impact polystyrene (HIPS), Urethanes, and custom materials.
Product design and performance parameters include:
Quick-turn prototyping is essential in medical device manufacturing today. Time to market is a top priority for medical device manufacturers—delivering functional prototypes into the hands of all stakeholders as soon as possible allows for more-informed decision-making and shorter time to market.
Lead times can be as short as:
Spectrum has constructed a state-of-the-art cleanroom for its additive manufacturing operations, which allows sensitive and/or mission-critical materials and components to be processed inside a cleanroom environment. The cleanroom houses additive manufacturing equipment and materials, including 12 production proprietary thermoplastic machines capable of running 24/7 and remotely monitored anywhere in the world. Applications vary from R&D prototypes to production-ready medical use parts, including in-human devices. The cleanroom is certified Class 7.
Bringing a medical device to market can take years and requires deep expertise in identifying product requirements and the best product design to meet clinical needs. Spectrum’s additive manufacturing capabilities allow medical-device designers to select from a wide range of medical-grade plastics with specifically engineered material characteristics and design components, including, multi-material and multi-dimensional builds.
For more information on AM, or to discuss if AM is a good choice for your next project, please contact us to speak directly with our technical experts.
We receive a wide variety of requests, ranging from "napkin-sketch" to highly detailed and specific designs and everything in between. We even had a customer design their part around 3D printing exclusively through us.
Any time is ideal for reaching out to the AM hub. Usually, customers benefit more early on as they can decide the materials, design, dimensions, etc., with quick iterations instead of going with a specific design right into larger-scale testing. However, the customer can contact the AM hub and find us beneficial at any point.
If you have a design to test out, whether it involves a tube or a 3D-printed component, feel free to contact us! We welcome challenges and constantly evolve and grow our hubs to create the next breakthrough product that better serves our customers.
Contamination by airborne articles into the materials as a device is being made can impact its quality and function, causing risk to the patient. For example, to maintain clean the air, ISO Class 7 requires 60-150 air changes per hour to filter out contaminants.
The FDA expects all medical-device companies and their contract manufacturers to follow Good Manufacturing Practices (GMP). Cleanrooms classification ranges from ISO Class 5 to ISO Class 8, depending on how the product is used and its sterility requirements.
We have a HEPA air filtration system, pressure and temperature controls, electrostatic control systems, and protocols for ensuring purity. We also meet all FDA requirements for cleanliness and care of materials, equipment, and tools.