The Extrusion of Acetal Core Mandrel at Six Sigma Quality Levels

The Extrusion of Acetal Core Mandrel at Six Sigma Quality Levels

Click here to download the white paper.
Braid reinforced medical tubing was invented by Norman C Jeckel in 1942 in Glens Falls, NY for US Catheter Corp. This concept was further developed in 1969 by Robert C. Stevens of Cordis Corporation to include continuous braiding and extrusion processes that utilize a removable core mandrel as a manufacture aid to construct braid reinforced angiographic catheters as shown below. Braided tubing includes a metal or polymer braid reinforcement encapsulated between two thin-wall thermoplastic elastomer layers to improve the kink resistance, torqueability, pushability and burst pressure of a catheter. The core mandrel is used to support and control the ID of the catheter tubing during the over-extrusion and braiding processes.
Over the years, not much has changed in the general manufacturing process for thin-wall braided tubing except that silver-plated copper core mandrel has mainly been replaced with lower cost Acetal, a high strength polymer that can be extruded into solid round core mandrel with a high degree of dimensional stability. Acetal is a good material choice because of its low coefficient of friction, it will not deform during the extrusion and braiding processes, and it will uniformly elongate when stretched from both ends for easy removal from the catheter.
Although the basic manufacturing concept has not changed substantially, extrusion technologies have indeed advanced and allowed for higher quality levels to support modern interventional technologies. Examples would include low-profile braid reinforced tubing for structural heart catheters and for electrophysiology catheters for cardiac mapping and the delivering of ablative energy to cardiac tissue.
Medical technology contract manufacturers need to continuously improve production efficiency to remain competitive. With increasingly tighter tolerance specifications driven by 6 Sigma quality initiatives, however, such efficiency gains are more challenging to maintain. Some level of variation pervades every extrusion process. Control of this variation is required for tight tolerance, nearly defect free extrusion. Maintaining a stable process on target dimensions and systematically reducing process variation are keys to achieving 6 Sigma targets.
High production yields require not only precision machined extrusion tooling and polymer lot to lot consistency, but also exceptional extrusion process optimization. Tight tolerance Acetal core mandrel extrusion is an intricate process and in order to keep the process under statistical control, there are certain variables such as melt temperature uniformity, head pressure stability, haul-off speed consistency, and winding tension levels that need to be precise and as stable as possible in order to achieve industry standard OD tolerances while guaranteeing 6 Sigma process capability.
At Spectrum Plastics Group, we look at process capability Cpk, and process performance Ppk indices to evaluate our Acetal core mandrel extrusion process output. Cpk accounts for the variation within subgroups but does not account for variation drifts between subgroups. Ppk accounts for the overall variation of all measurements taken including variation within subgroups and between them.
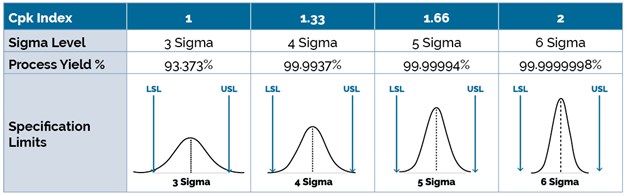
The relationships between the Cpk indices, sigma levels, process yields, and upper & lower specification limits (USL, LSL) are shown in the table below. If Cpk equals to 1 or 3 Sigma, it means that process has high risk and it could be out of control at any time. A Cpk of 1.33 or 4 Sigma is the typical target for medical device components yet the medical device industry is pushing towards achieving a Cpk of 1.66 or 5 Sigma. If Cpk is 2, it means the process is under control and at a 6 Sigma level. Targeting a 6 Sigma level is essentially aiming for a process that is 99.9997% free of defects.
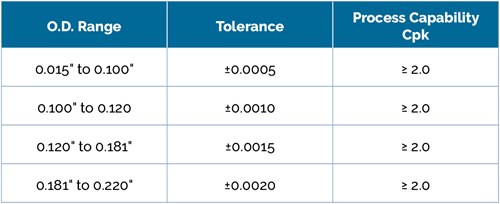
Through extensive process optimization and design of experiments (DOE), Spectrum Plastics offers Acetal core mandrel with guaranteed tolerance levels. Our process capability indices are shown in the table to the right:
Spectrum Plastics combines multi-layer extrusion technology with 6 sigma methodologies to repeatablely deliver the highest quality acetal core mandrel used for braid reinforced interventional catheters. Our proprietary Acetal silicone blend may be used on the outer layer of the core mandrel for enhanced frictional properties resulting in a lower release force when removing the mandrel from the catheter.
Level winding and winding tension are important factors for high-precision winding of Acetal core mandrel products. When unwinding the core mandrel into the over-extrusion and braiding processes, each revolution will produce tight and slack tension waves. These tension waves are amplified and cause variations in the over-extrusion and braiding processes such as inconsistent pick count and poor spacing between the braid wires. Spectrum Plastics’ extrusion technology incorporates near tensionless, level winding of Acetal core mandrel onto spools. Level winding as shown on the below picture maximizes the amount of core mandrel on the spool and assists with stable unwinding of the core mandrel into subsequent extrusion and braiding processes.